What Are Covalent Compounds Made Up Of
Molecules can have different shapes. Properties of Ionic and Covalent Compounds.
Covalent Compounds Covalent Bond Properties Examples With Videos
In chemistry a hydride is formally the anion of hydrogen H.
What are covalent compounds made up of. The law of constant composition can be used to distinguish between compounds and mixtures of elements. If the prefix of the first element would be mono- it is not neededTIP. Compounds have a constant composition.
Pure Substance Definition in Chemistry. Two atoms sharing a pair of electrons. A perfect diamond is a single molecule made of carbon atoms.
Mixtures do notWater is always 888 O and 112 H by. The term is applied loosely. Get used to what part of an elements.
Water H 2 O is a hydride of oxygen ammonia is a hydride of nitrogen etcFor inorganic chemists hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. Water molecules are not absolutely neutral and have a slight negative charge on the oxygen atom and slight positive charges on the hydrogen atoms and since covalent compounds are made up of neutral molecules or molecules with slight charges and hence are not attracted to water molecules strongly. At one extreme all compounds containing covalently bound H atoms are called hydrides.
Second look at the subscript of each element to determine which prefix to use. For many molecules the sharing of electrons allows each atom to attain the equivalent of a full valence. Discover unexplored unique chemotypes with our MADE collection.
MAke-on-DEmand MADE building blocks are a 250M-catalog of reagents that can be synthesized within several weeks using short pre-validated reaction sequences our. Ionic bond type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Learn more about ionic bonds in this article.
Therefore these compounds are insoluble in water. Steps to Naming Covalent Compounds. First identify the elements present.
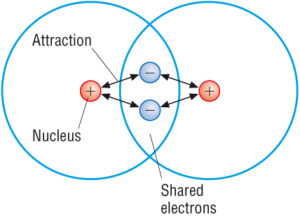
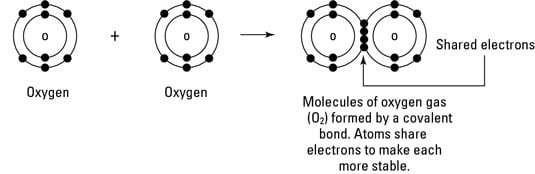
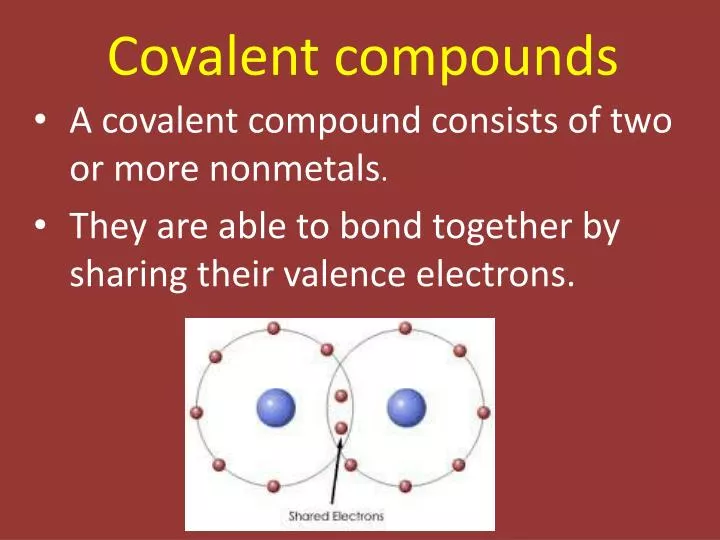
A covalent bond is a chemical bond that involves the sharing of electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. When atoms approach one another their electrons interact and tend to distribute themselves in space so that the total energy is lower than it would be in any alternative arrangement. Here is a list of examples of compounds made of two elements.
Names and Uses of 10 Common Gases. Examples of compounds include table salt or sodium chloride NaCl an ionic compound sucrose a molecule nitrogen gas N 2 a covalent molecule a sample of copper intermetallic and water H 2 O a covalent molecule. Chemical bonding any of the interactions that account for the association of atoms into molecules ions crystals and other species.
If an element does not have a prefix assume that the subscript is 1 Third apply the above naming scheme. Examples of Compounds. These shared electrons are found.
Such a bond forms when the valence outermost electrons of one atom are transferred permanently to another atom. A covalent bond is a shared pair of electrons between two non-metal atoms for example carbon dioxide. A covalent bond is formed when two atoms share a pair of electrons.
The law of constant composition states that the ratio by mass of the elements in a chemical compound is always the same regardless of the source of the compound. 66 of the mass of the human body is made up of oxygen atoms. A compound is made up of two or more elements chemically bonded together.
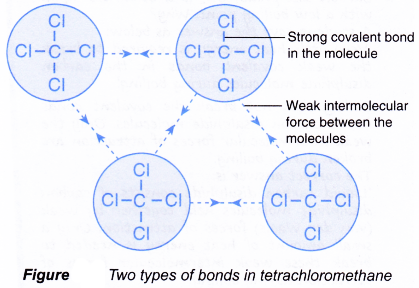
Covalent bonding occurs in most non-metal elements and in compounds formed between non-metals. Organic compounds are compounds that contain carbon. Some are long spirals while others may be pyramid shaped.

Covalent Compounds Covalent Compound Electrons Are Shared Between 2 Different Atoms 1 Bond Forms Between Two Elements That Each Share One Electron Ppt Download

Covalent Bond Definition Types And Examples

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Naming Covalent Compounds Nomenclature Rules

Covalent Bond Examples Formation Properties What Is A Covalent Bond Video Lesson Transcript Study Com

How To Name Ionic And Covalent Compounds Chemistrynotes Com

Covalent Bonds Biology For Majors I

Covalent Bond Biology Dictionary

Covalent Compounds Covalent Bond Properties Examples With Videos

Covalent Bonding Biology Definition Role Expii
Environmental Science What Is Covalent Bonding Dummies
Ppt Covalent Compounds Powerpoint Presentation Free Download Id 5168896

Covalent Bond Definition Types And Examples
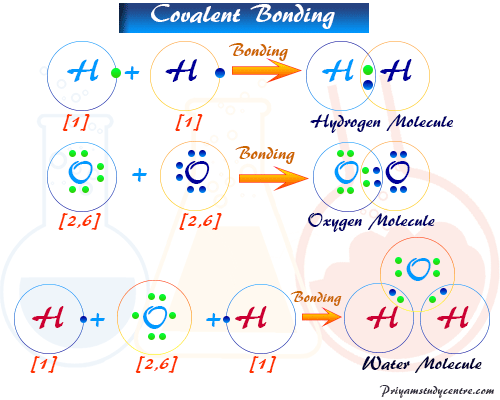
Covalent Bond Types Definition Properties Examples
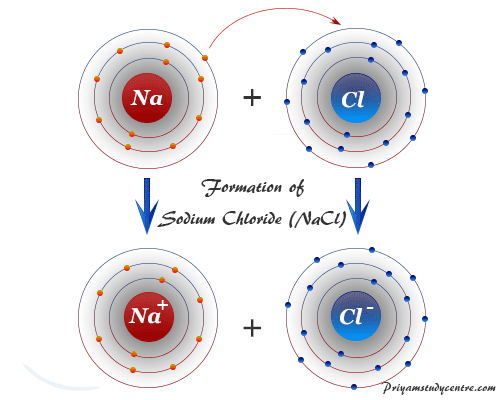
Covalent Bond Types Definition Properties Examples

Covalent Bond Examples Science Struck

Properties Of Ionic And Covalent Compounds A Plus Topper

Covalent Bond Definition Properties Examples Facts Britannica
Which Are Soluble In Water Covalent Compounds Or Ionic Compounds Quora