Is Remdesivir Effective For Ebola
National Institute of Allergy and Infectious Diseases NIAID and the Ministry of Health in Liberiaone of the hardest-hit countriesestablished PREVAIL or the Partnership for. Is Remdesivir Effective Against COVID-19 Based on the Limited Evidence Available.

Us Regulators Approve 1st Treatment For Ebola Virus
Fauci claims that Remdesivir was effective in treating Ebola in scientific studies a few years ago.

Is remdesivir effective for ebola. Between March 22 2020 and Jan 21 2021 857 participants were enrolled and randomly assigned to remdesivir plus standard of care n429 or standard of care only n428. Remdesivir for Covid-19 Final Report In this randomized double-blind trial in 1062 adults hospitalized with Covid-19 remdesivir was superior to placebo in shortening the time to recovery 10. 6 Remdesivir is an adenosine analogue which incorporates into nascent viral RNA chains and results in.
In fact Remdesivir killed 54 percent of the people according to the studys own statistics. 3 Actualmente se encuentra en estudios experimentales con pacientes con COVID-19 no obstante su uso incrementa el riesgo. It is currently under clinical development for the treatment of Ebola virus infection.
But it showed promise against other viruses. Remdesivir código de desarrollo GS-5734 1 es un medicamento antiviral un nuevo profármaco 2 usado por primera vez en 2009 que pertenece al grupo de los análogos de nucleótidosLa vía de administración es mediante inyección intravenosa y oral en infusión. Despite the availability of effective SARS-CoV-2 vaccines improving care for patients with symptomatic infection remains relevant.
It was quickly pushed through clinical trials during the West African outbreak of 2013-15 after showing promising results in the lab and was then rolled out more widely in affected areas of Africa. Remdesivir the active drug in Veklury was originally developed for the treatment of Ebola. The truth is that Remdesivir failed so badly that it was discontinued and did not even finish the study.
The 2014-2016 Ebola outbreak in West Africa was the largest in history with nearly 28700 cases and more than 11300 deaths. Remdesivir exhibits broad in vitro antiviral action against zoonotic and human pathogens from multiple virus families Table 1Remdesivirs activity has been consistent when tested against members of the Filoviridae Paramyxoviridae Pneumoviridae and Coronaviridae. Remdesivir has broad-spectrum activity against members of several virus families including filoviruses eg Ebola and coronaviruses eg.
Strategies to blunt the hyperinflammatory state that characterises severe COVID-19 include broad-spectrum immunosuppressive drugs such as corticosteroids targeted immunomodulatory treatments such as tocilizumab or baricitinib and direct. Remdesivir wasnt an effective treatment for either disease. Other investigational medications worked better but it was shown to be safe for patients.
In that study Veklury remdesivir was compared to three other treatments for Ebola. Remdesivir is an experimental antiviral drug produced by the US pharmaceutical company Gilead initially as a potential treatment for Ebola. Remdesivir has demonstrated broad-spectrum antiviral activity against RNA viruses belonging to different families including Paramyxoviridae respiratory syncytial virus Nipah virus and Hendra virus Coronaviridae SARS-CoV MERS-CoV and bat coronaviruses as well as Filoviridae Ebola virus in vitro and in preclinical studies 6 In particular in nonhuman primates inoculated with.
Numerous clinical trials have been initiated to identify an effective treatment. 6 Among HCoV remdesivir inhibits three of the endemic strains associated with respiratory illness HCoV. Remdesivir was tested in a.
Remdesivir which was originally developed as a treatment for Ebola and hepatitis C interferes with the reproduction of viruses by jamming itself into new viral genes. That study was stopped before it was concluded because of a significant increase in mortality in patients taking RDV meaning it didnt help those Ebola. The first was a study of its use against the Ebola virus in 2019.
One leading candidate is remdesivir GS-5734 a broad-spectrum antiviral that was initially developed for the treatment of Ebola virus EBOV. During the COVID-19 pandemic remdesivir was approved or authorized for emergency use to treat COVID19 in around 50 countries. Remdesivir in a syringe.
There is only one randomized control trial for remdesivir RDV conducted during the last Ebola outbreak. After results from the first 499 participants had been reviewed the trials safety monitors recommended that two drugsZMapp and remdesivirbe dropped from the remainder of the trial. It is administered via injection into a vein.
As local and international healthcare workers responded to the outbreak the US. The control group was not actually a placebo group. Both molecules were effective so Pedersen decided to pursue the simpler one GS-441524.
Remdesivir sold under the brand name Veklury is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. Studies in cells and animals suggested that remdesivir was effective. Ardis says that Remdesivir was by far the least safe and effective of the four drugs in the Ebola trial and that the one-year study was canceled by safety monitors in August of 2019 after just six months because of a 531 mortality rate not from ineffectively treated Ebola but from kidney and other organ failure.
This is a rare but serious disease thats caused by a viral infection. While clinical trials suggest remdesivir isnt very effective in treating Covid-19 recent studies have shown that it does block Coronavirus activity. The virus that causes Ebola is not a coronavirus but remdesivir is.
Before the COVID-19 pandemic research already suggested that remdesivir might be effective at fighting coronaviruses. Updated guidelines from the World Health Organization in November 2020. 15 participants were excluded from analysis in the remdesivir group and ten in the control group.
These two drugs were much less effective at preventing death. FDA has issued emergency use authorization for the investigational antiviral drug remdesivir for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized. Remdesivir is owned by Gilead Sciences a US biotechnology company.
Although remdesivir performed well in preclinical studies it did not meet efficacy endpoints in a randomized trial. Researchers tested remdesivir in clinical trials during the Ebola outbreak. Remdesivir Proven Ineffective in Ebola Study.
The drug was originally developed for Ebola but has shown promise with COVID-19. And Ars Technica Addendum effective. There was only one other human study cited and the results were devastating for the patients in that study who were administered Veklury remdesivir.
Overall about 50 of people who received either Zmapp or remdesivir died during the trial. Studies in animals have shown potential and some human studies including in some studies treating Ebola have indicated relative safety.

How To Bring Down The Price Of Drugs Such As The Novel Coronavirus Therapy Remdesivir Equitable Growth

What Is Remdesivir How Is It Used For Covid 19 Covid 19 Featured Health Topics Hackensack Meridian Health

Remdesivir Price Still A Puzzle To Be Solved By Gilead Sciences Shots Health News Npr

Remdesivir No Evidence Drug Trump Took For Coronavirus Is Effective Treatment Says Who The Independent

Remdesivir Drug Used For Ebola Might Be Effective Against Covid 19 Icmr The Wire Science
Racgp Remdesivir And Covid 19 What Gps Need To Know
Remdesivir Has Little Effect On Covid 19 Mortality Who Study Says Financial Times

Gilead S Coronavirus Drug Remdesivir What To Know About The Experimental Treatment Cbs News

Covid 19 Drug Shortage How Sudden Spike In Cases Resulted In Shortfall Consequent Ban On Remdesivir Exports The Financial Express
Remdesivir Results Promising In Medical Center Clinical Trial Newscenter

When Do You Really Need Remdesivir Does It Work
/cdn.vox-cdn.com/uploads/chorus_asset/file/19854140/Visual_Inspection___Investigational_Remdesivir.jpg)
Uic Testing Antiviral Drug Against The Coronavirus As Hospital Sees Surge Of Cases Chicago Sun Times

Remdesivir S Fda Approval To Treat Covid 19 Sets It Ahead Of Treatment Pack

Remdesivir Connecticut College News

Why Haven T We Seen The Data On Remdesivir

Remdesivir What Is The Repurposed Covid 19 Drug Why Is There A Shortage Do You Need It Coronavirus Outbreak News

Skip To Main Content Connecticut College Connections Navigation Search Connecticut College Close Current Issue Past Issues 2021 Issues 2020 Issues 2019 Issues 2018 Issues 2017 Issues 2016 Issues 2015 Issues 2014 Issues Older Issues

Remdesivir Coronavirus Trial Issues In China Give Warning To Other Countries
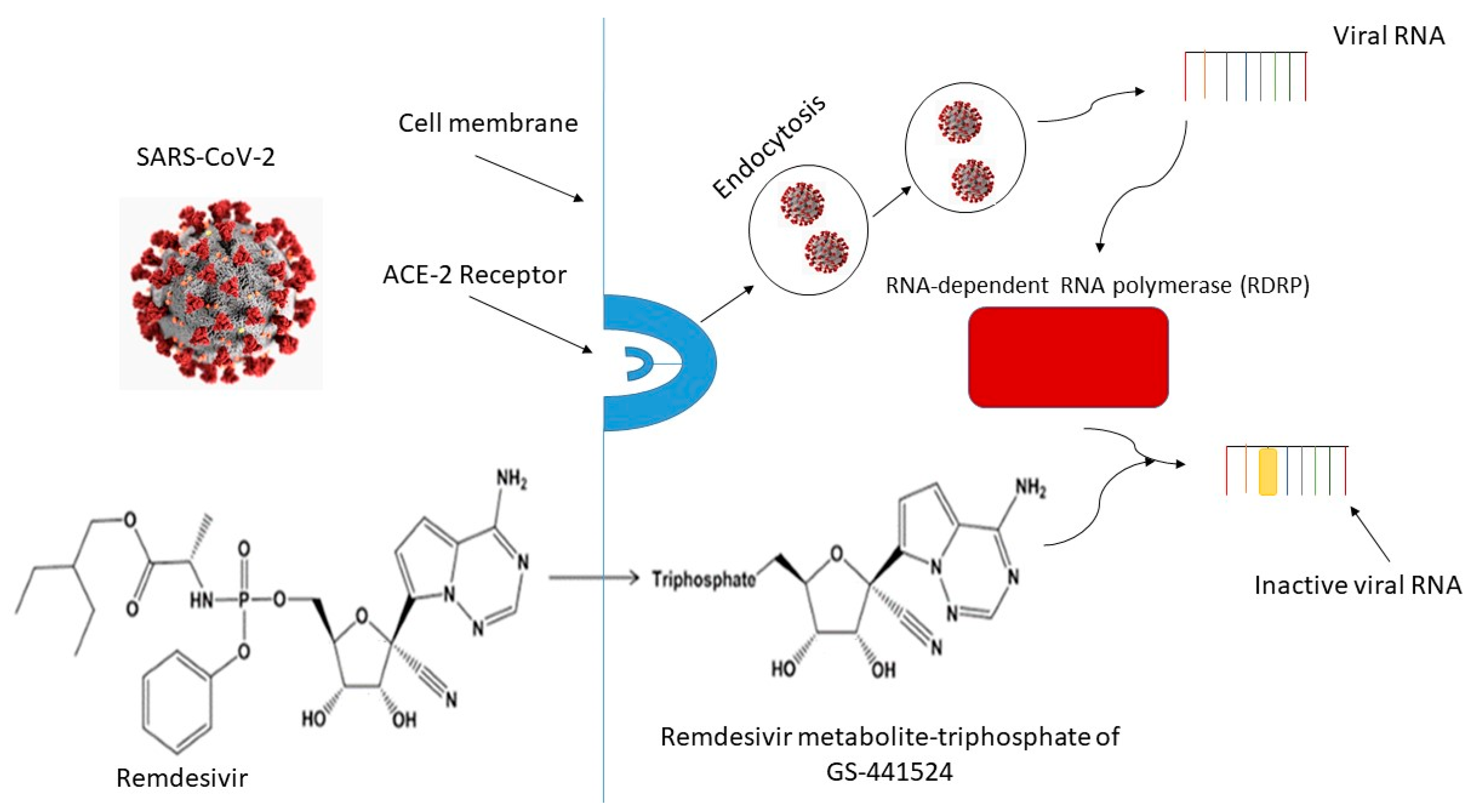
Sci Pharm Free Full Text Remdesivir Bringing Hope For Covid 19 Treatment Html