What Covalent Bonds Are Most Polar
A The electrons in the covalent bond are equally shared by both hydrogen atoms. Only d is true.
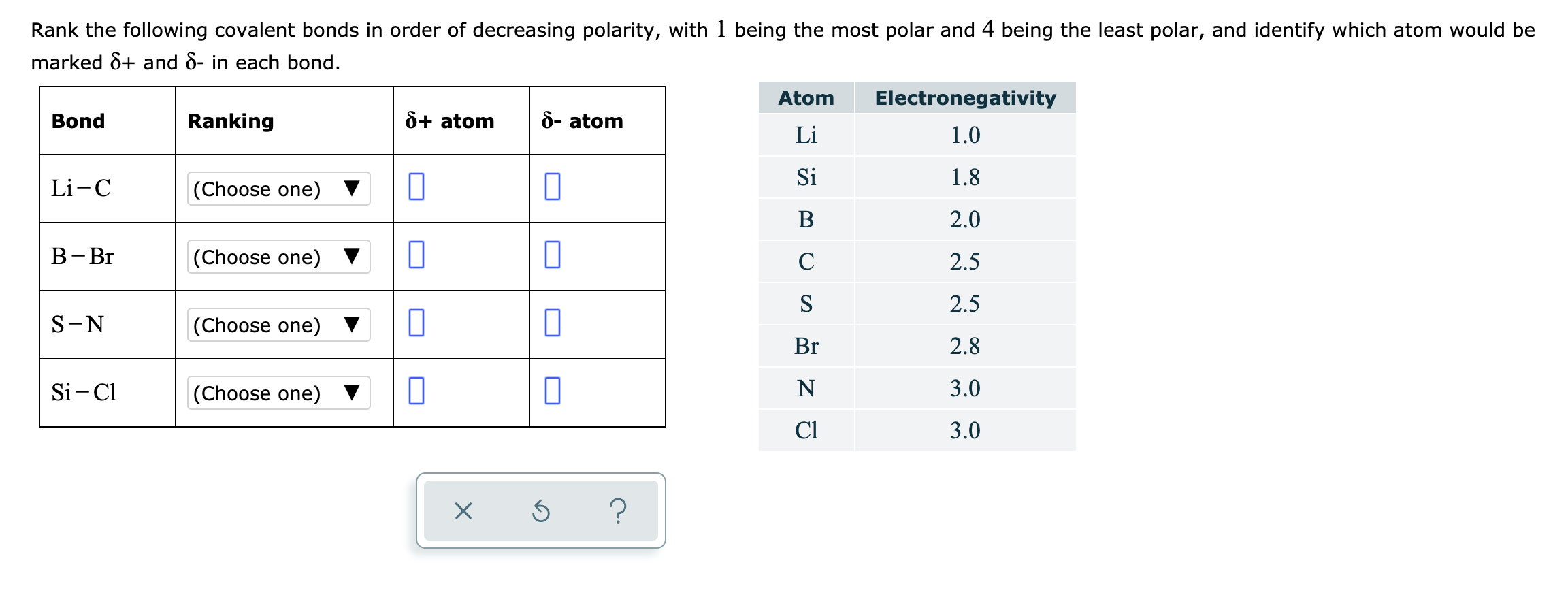
Rank The Bonds From Most Polar To Least Polar A C O C F C N B C Ci C I C Br C H O H N H C D C H C C C N Study Com
However a molecule may be polar or nonpolar depending on its geometry.

What covalent bonds are most polar. This is a nonpolar covalent bond. Net Dipole Moments Molecules as a whole are often polar from vector summation of individual bond polarities and loneindividual bond polarities and lone-pair contributionspair contributions Strongly polar substances soluble in polar solvents like water. H 2 N 2 O 2 etc.
The bonding electrons in polar covalent bonds are not shared equally and a bond moment results. The bully child seems to spend more time playing with the toy than the other child. Covalent bonds in which the sharing of the electron pair is unequal with the electrons spending more time around the more nonmetallic atom are called polar covalent bonds.
In these cases the electrons are still considered shared that is the bond is still considered covalent but the sharing is not perfect. Lewis theory Gilbert Newton Lewis 1875-1946 focuses on the valence electrons since the outermost electrons are the ones that are highest in energy and farthest from the nucleus and are therefore the ones that are most exposed to other atoms when. It is a bonding between atoms within a molecule and forms the strongest bonds anywhere.
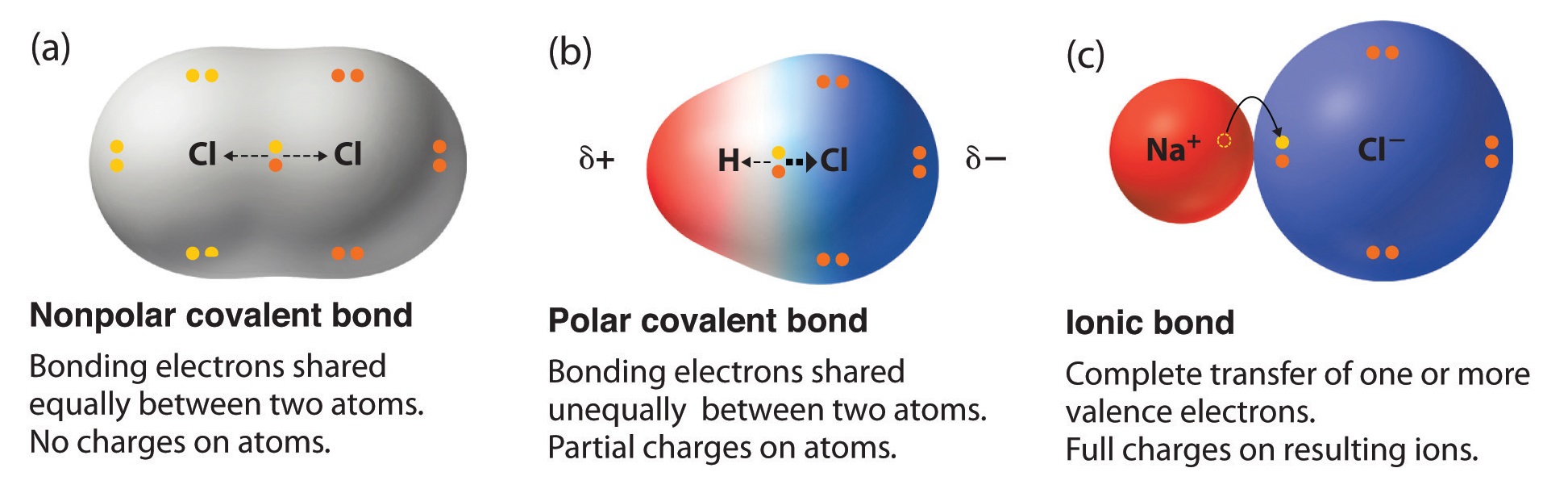
A covalent bond is a chemical bond that involves the sharing of electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. A molecule is nonpolar if the shared electrons are are equally shared. The terms polar and nonpolar are usually applied to covalent bonds that is bonds where the polarity is not complete.
B The fluorine atom attracts the electrons in the bond more than the hydrogen atom does leading to. A covalent bond is formed when atoms share valence electrons. A completely polar bond is more correctly called an ionic bond and occurs when the difference between electronegativities is large enough that one atom actually takes an electron from the other.
Covalent bonds are the most common and most important kind of bonding. This partial ionic character of covalent bonds increases with the difference in the electronegativities of the two. Figure 45 Polar versus Nonpolar Covalent Bonds.
Ionic Bonds Finally for atoms with the largest electronegativity differences such as metals bonding with nonmetals the bonding interaction is called ionic and the valence electrons are typically represented as being. Polar covalent is the intermediate type of bonding between the two extremes. To get a polar covalent bond the difference in EN between hydrogen and the other atom it bonds with must be greater than 05 and smaller than 17 - 18.
Ionic and covalent bonds are the two extremes of bonding. Again polar covalent bonds tend to occur between non-metals. Compounds with covalent bonds usually have lower enthalpies of.
There are two types of covalent bonds. Covalent compounds can be either polar or nonpolar but they contain weaker bonds than ionic compounds because they. To determine the polarity.
Polar Covalent Bonds. For many molecules the sharing of electrons allows each atom to attain the equivalent of a full valence. Each nitrogen atom is able to share three electrons for a total of.
This form of chemical bonding is typical in carbon-based compounds. Methane gas CH 4 has a nonpolar covalent bond because it is a gas. Match each atom or molecule with its corresponding.
Polar covalent bonds are made by two atoms with different electronegativities but the different should not be exceeding 17. The atoms do not always share the electrons equally so a polar covalent bond may be the. For example tetrachloro-methane carbon tetrachloride CCl 4 has polar CCl bonds but the tetrahedral arrangement of the four bonds about the central carbon atom causes the individual bond moments to cancel.
As this bond is formed between two same atoms the difference in electronegativity is zero thus they both attract bonding electron pair equally and no polarity is found within the molecule. Covalent bonds are directional where the atoms that are bonded showcase specific orientations relative to one another. A molecule is polar if the shared electrons are equally shared.
Nonpolar substances are insoluble in water. Electronegativity differences in bonding using the Pauling scale. Classifying bonds as covalent polar covalent or ionic.
The two main types of bonds formed between atoms are ionic bonds and covalent bonds. The most common gas in the atmosphere nitrogen is made of two nitrogen atoms bonded by a triple bond. In polar covalent electron pair is pulled more by one atom compared to the other atom.
The sharing of electrons between atoms is referred to as a covalent bond. The pair of electrons which are shared by the two atoms now extend around the nuclei of atoms leading to the creation of a molecule. Covalent bonds rarely break spontaneously after it is formed.
Covalent bonds between identical atoms as in H 2 are nonpolarie electrically uniformwhile those between unlike atoms are polarie one atom is slightly negatively charged and the other is slightly positively charged. Ionic compounds dissolve in polar solvents like water stack neatly on each other to form crystals and require a lot of energy for their chemical bonds to break. Some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic.
This electrostatic attraction between the two poles dipoles is much weaker than ionic or covalent bonding so molecular solids tend to be softer than ionic crystals and have lower melting points. A non polar covalent bond is formed when two same atoms share electrons by head to head overlapping single covalent bond. To be nonpolar covalent bonds so somewhere in between there must be the difference between nonpolar covalent bond and a polar covalent bond and most textbooks will tell you approximately somewhere in the 05 range so.
In some cases three covalent bonds can be formed between two atoms. In such a bond there is a charge separation with one atom being slightly more positive and the other more negative ie the bond will produce a dipole moment. In non-polar covalent bonds electrons are equally shared by the two atoms participating in making the bond.
However most covalent bonds occur between elements where even though the electronegativity difference is lower than 17 it is not zero. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. Covalent bonds are chemical bonds between two non-metal atoms.
In a polar covalent bond shown in Figure 1 the electrons are unequally shared by the atoms and are attracted more to one nucleus than the otherBecause of the unequal distribution of electrons between the atoms of different elements a slightly positive δ or slightly negative δ charge develops. Covalent bonds result from a sharing of electrons by two or more atoms usually nonmetals. For example most carbon-based compounds are covalently bonded but can also be partially ionic.
Most compounds having covalent bonds exhibit relatively low melting points and boiling points. According to the Pauling scale hydrogen has an electronegativity EN value of 220. The Octet rule only applys to molecules with covalent bonds.
Have you ever seen two children play and one child acts like a bully toward the other child. A covalent bond between atoms is formed when they share one or more pairs of electrons among each other.
Solved Question 1 Which Molecule Has The Most Polar Covalent Chegg Com
8 7 Bond Polarity And Electronegativity Chi Chemistry Libretexts

Ionic Bonds Polar Covalent Bonds And Nonpolar Covalent Bonds Youtube

Polar Covalent Bond An Overview Sciencedirect Topics

Polar Covalent Bond Definition And Examples

Polar Covalent Bond Definition And Examples
Solved Rank The Following Covalent Bonds In Order Of Chegg Com

Which Of The Following Bonds Will Be Most Polar Youtube

Which Covalent Bond Is The Most Polar A C F B
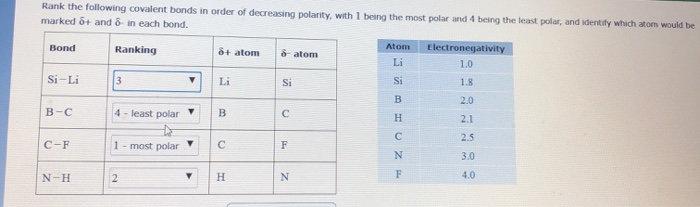
Solved Rank The Following Covalent Bonds In Order Of Chegg Com

Solved 4 Circle The Most Polar Bond From Each Set Of Chegg Com
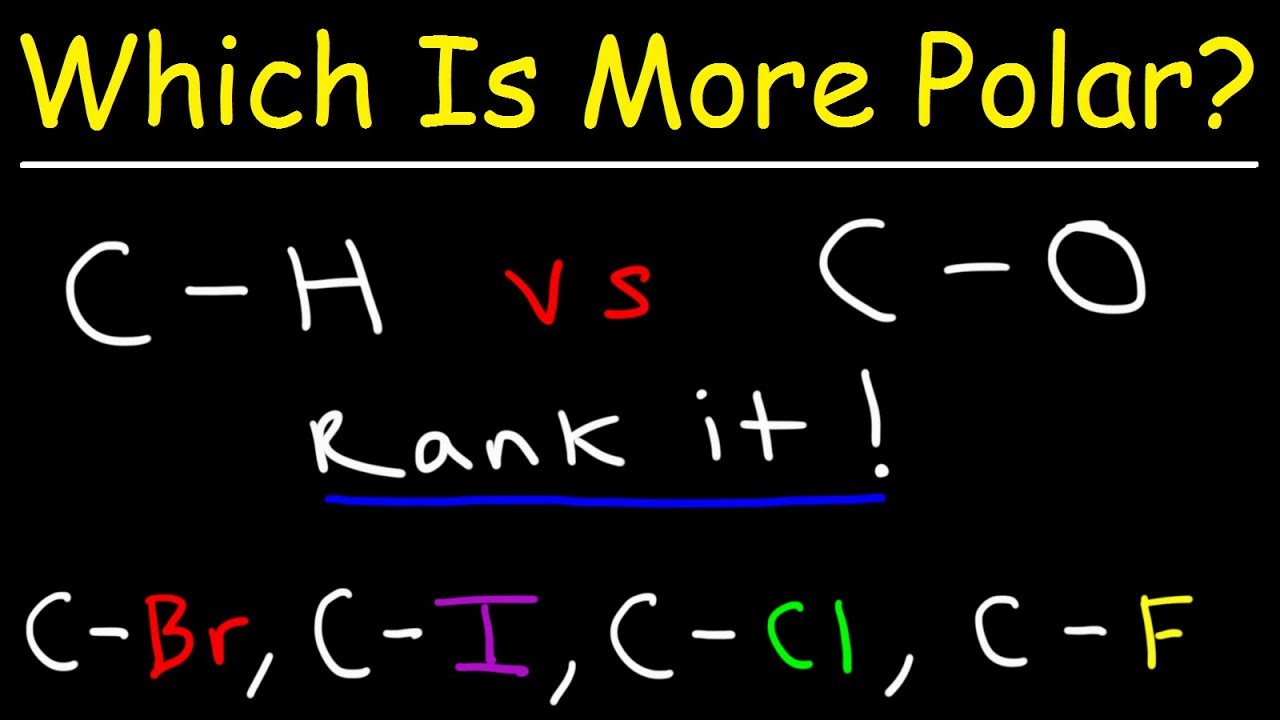
Which Bond Is More Polar Youtube

Polar Vs Non Polar Bonds Molecules Chemtalk

Polar Covalent Bond Definition And Examples
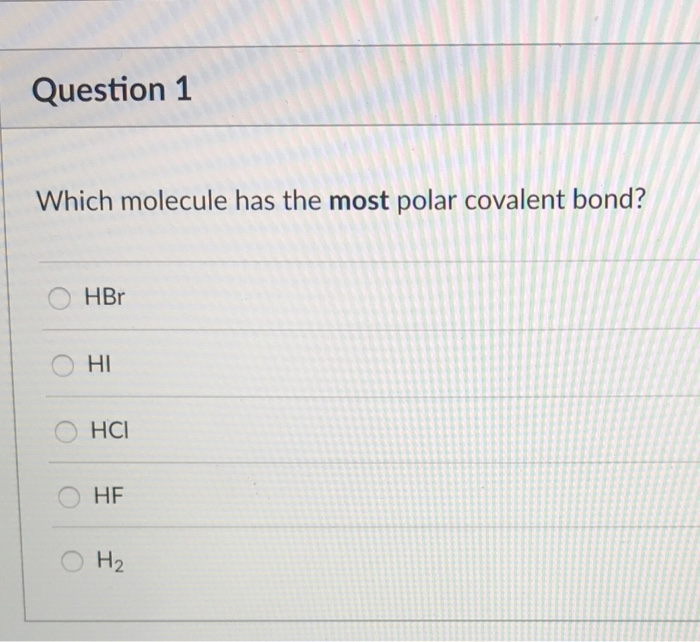
Solved Question 1 Which Molecule Has The Most Polar Covalent Chegg Com

Polar And Nonpolar Covalent Bonds Definitions Molecules And Examples

Covalent Bonds Biology For Majors I
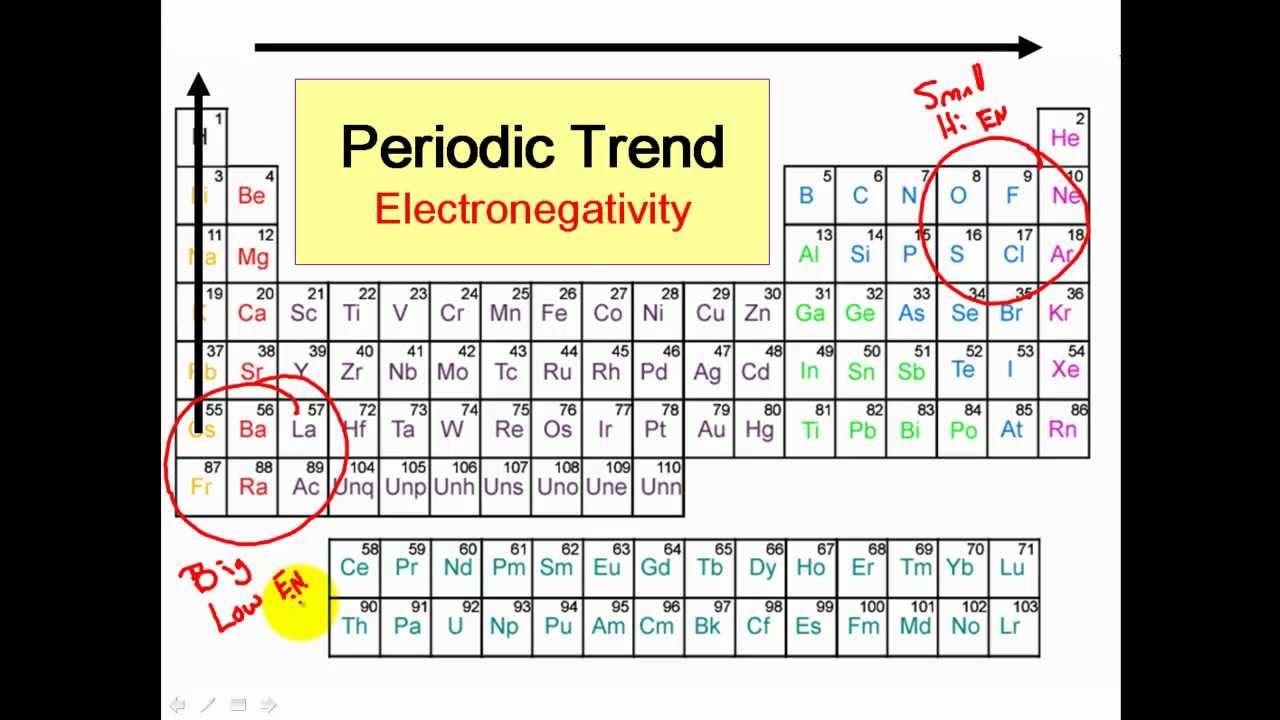
Polar And Nonpolar Covalent Bonds Clear Simple Youtube
