Which Organic Molecule Has The Most Energy
Oxygen is more electronegative than carbon so it wants to hold the electrons of the covalent bond more than. Thus when breaking a chemical bond energy needs to be added to overcome the bond energy between the two atoms.
Each molecule has its own characteristic bond energy.

Which organic molecule has the most energy. All organic molecules contain carbon and the ability to manipulate carbon bonds was probably a very early development in the evolution of life. Short range repulsion only matters when atoms are in very close proximity R d i but at. The large exponent means that when R d i then small decreases in R cause large increases in repulsion.
By itself Infrared IR spectroscopy isnt a great technique for solving the structure of an unknown moleculeHowever weve seen that IR spectroscopy can a great technique for identifying certain functional groups in an unknown molecule especially functional groups containing OH or CO. Means that by knowing the structure of a molecule we should be able to predict its function such as its solubility acidity or basicity stability reactivity towards a particular species such as H etc. When new bonds form they release energy.
The electron is boosted to a higher energy state and attached to a primary electron acceptor which begins a series of redox reactions passing the electron through a series of electron carriers eventually attaching it to a molecule in Photosystem I. In the following diagram two 1s atomic orbitals combine to give a sigma σ bonding low energy molecular orbital and a second higher energy MO referred to as an antibonding orbital. Major histocompatibility complex MHC.
Carbohydrates particularly glucose lipids and protein are the most commonly oxidized compounds. Orbitals Shell s p d f Total Electrons Possible 1 1 2 2 2 3 8 3 3 3 5 18 4 1 3 5 7 32 energy level 1 contains up to two electrons in a spherical orbital called a 1s orbital. Predicting the solubility of an organic molecule is a very useful skill.
Organic chemistry is a branch of chemistry that studies the structure properties and reactions of organic compounds which contain carbon in covalent bonding. The coronavirus disease 2019 COVID-19 pandemic has highlighted the need for rapid and sensitive protein detection and quantification in simple and robust formats for widespread point-of. In the kinetic theory of gases the term molecule is often used.
It has 4 pi bonds and a single pair of electrons on the nitrogen that participates in the pi system giving 10 pi electrons in total. Look it up now. A French chemist by name Anselme Payen was.
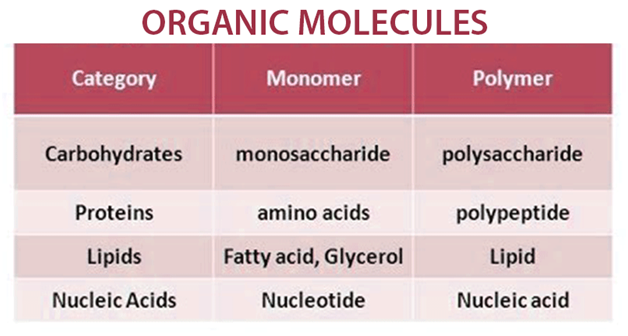
In quantum physics organic chemistry and biochemistry the distinction from ions is dropped and molecule is often used when referring to polyatomic ions. Carbohydrates are organic compounds made of the elements carbon hydrogen and oxygen. Four major classes of biologically important organic molecules.
The ratio of hydrogen atoms to oxygen atoms in carbohydrate molecules is 21. The bonding MO is occupied by two electrons of opposite spin the result being a covalent bond. The compound must follow Hückels Rule the ring has to contain 4n2 p-orbital electrons.
The hydrogen molecule provides a simple example of MO formation. Every atom around the perimeter of the two rings participates in the pi system. For example most reactions are carried out in.
Light acts on a molecule of P700 in Photosystem I causing an electron to be boosted to a. Cellulose is a complex carbohydrate consisting of oxygen carbon and hydrogen. Organisms use carbohydrates as energy sources structural units and for other purposes.
The repulsive energy goes up as d i R 12 where R is the distance between the atoms and d i is the distance threshold below which the energy becomes repulsive. The electronegativity of carbon is too small for carbon to gain electrons from most elements to form C 4-ions and too large for carbon to lose electrons to form C 4 ions. Biologic oxidation of these organic compounds by bacteria results in.
Carbon is probably the most important element for all living organisms. For the first two energy levels shells 1 and 2 are the most important for bonding in organic chemistry. A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.
Carbon-based molecules get special attention because no other element comes close to carbons versatility. Among the many distinctive features of benzene its aromaticity is the major contributor to. Heterotrophic bacteria which include all pathogens obtain energy from oxidation of organic compounds.
CarbonChemists refer to most molecules that contain one or more carbon atoms as organic. Phagocytic cell derived from blood monocytes typically resident in most tissues. Carbon has a unique ability to form 4 covalent bonds which can lead to long chains of molecules.
The study of organic reactions includes the. Indole is a bicylic molecule that looks like a molecule of benzene fused to a molecule of pyrrole. What are the major types of organic molecules.
In this molecule a electronegative atom O is covalently attached to a carbon. It has both scavenger and antigen-presenting functions in immune responses. Carbon therefore forms covalent bonds with a large number of other elements.
Carbon has four valence electrons 2s 2 2p 2 and it must either gain four electrons or lose four electrons to reach a rare-gas configuration. The previous figure has a scheme of the most typical electrophile in organic chemistry. Study of structure determines their structural formulaStudy of properties includes physical and chemical properties and evaluation of chemical reactivity to understand their behavior.
Carbohydrates are the largest class of organic compounds found in organisms. Molecule such as a protein nucleic acid or polysaccharide with a molecular mass greater than a few thousand daltons. Molecules are distinguished from ions by their lack of electrical charge.
For instance in an earlier post on the structure. It is chiral tasteless and has no odour. The study of these molecules is organic chemistry.
Out of 118 elements only one has its own field of study. This is a great example of how electronics work. IR Spectroscopy Practice Problems.
D i depends on the types of atoms. Chemical bonds between atoms store energy known as bond energy. Carbohydrates lipids proteins and related compounds nucleic acids and related compounds For each organic molecule class.
This means that the ring cannot contain a neutral sp 3 carbon. Each element within the ring must have a p-orbital that is perpendicular to the ring hence the molecule is planar. The compound must be cyclic.
Cellulose is the most abundant organic compound on earth with a chemical formula C 6 H 10 O 5n.
Organic Compounds Read Biology Ck 12 Foundation

Organic Vs Inorganic Molecules Definition Overview Expii

Organic Molecules Microbiology

Chemistry Ii Water And Organic Molecules

Chemistry Ii Water And Organic Molecules

Organic Molecules Microbiology
Organic Molecules Types Of Organic Molecules
Ch105 Chapter 5 Introduction To Organic Chemistry Chemistry
Absorbing Light With Organic Molecules

Classes Of Organic Compounds Boundless Chemistry
Drawing Organic Molecules Organic Chemistry Help

Chemistry Ii Water And Organic Molecules

Organic Molecules Microbiology

Organic Molecules Microbiology





